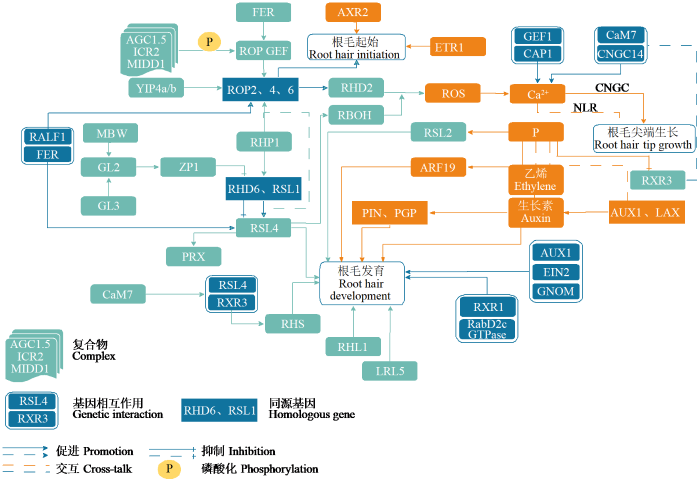
根毛是由根成熟区表皮细胞生长出的管状突起,是植物根系吸收矿质元素、水分以及与土壤微生物进行相互作用的主要器官。大多数双子叶植物、单子叶植物和蕨类植物根的表皮细胞都可能发育成为根毛。玉米(Zea mays L.)和水稻(Oryza sative L.)属于单子叶植物,其表皮细胞中只有短表皮细胞才能分化成根毛。拟南芥(Arabidopsis thaliana L.)中根毛表皮分为成毛细胞(trichoblasts)和非毛细胞(atrichoblasts),只有与2个皮层细胞相连接的表皮细胞(H型)才能发育形成根毛,该表皮细胞称为生毛细胞,而仅与一个皮层细胞相连的表皮细胞(N型)不能形成根毛[1-2]。根毛发育可分为4个重要阶段,即根毛细胞命运决定、根毛起始、根毛尖端生长和根毛成熟。这4个阶段涉及包括RHP1-Rop-RHD2-ROS-Ca2+调控根毛尖端生长途径、GL2-ZP1-RHD6转录因子调控途径、调控胞质信号分子的CNGC离子通道对根毛尖端Ca2+信号调控途径、载体介导的生长素调控根毛发育途径,研究大多集中在根毛起始和根毛尖端生长,对根毛细胞命运决定和根毛成熟的研究较少。本文从小G蛋白Rop调控途径、转录因子相关基因调控途径、Ca2+信号及相关激酶调控途径、磷酸盐(Pi)浓度调控途径和生长素(Auxin)运输及信号途径等方面对根毛生长发育及分子调控机制(图1)进行综述。
图1
图1
根毛生长发育基因调控网络
Fig.1
Regulatory network of root hair growth development genes
1 Rop调控途径
根毛由位于根尖端成熟区的成毛细胞发育而来,并以极性化的方式扩张生长,这种生长方式称为尖端生长。小G蛋白(small GTPase protein)Rop(Rho-type GTPase of plant)是植物中Rho小GTP结合蛋白的特异性亚家族。Rop定位于产生根毛的成毛细胞和根毛尖端的质膜[3-4],Rop参与调控根毛生长发育并在生长素、脱落酸(ABA)等植物激素介导的信号通路中起作用。拟南芥中,Ypt属于小G蛋白Rab家族,Ypt互作蛋白4a/b(YPT INTERACTING PROTEINS 4a/b,YIP4a/b)定位于反式高尔基体网络(trans-Golgi network,TGN)[5]。YIP4a/b参与蛋白质和细胞壁成分的分泌运输,有助于成毛细胞形成之前ROP2/4/6的激活和质膜积累。拟南芥中ROP2作为根毛生长的分子开关,决定着根毛起始;过表达ROP2能促进根毛尖端生长[3,6]。类固醇分子RHP1(ROOT HAIR PROMOTING AGENT1)促进根毛长度增加,且不会显著影响其发育进程。RHP1作用于Rop引导的局部信号通路促进根毛尖端生长,同样作用于RHD6(ROOT HAIR DEFECTIVE6)-RSL4(RHD6-LIKE4)依赖的转录通路上游[7]。根瘤形成因子(Nod factor,NF)能使根毛变形,导致根毛膨胀和弯曲。日本百合(Lilium japonicum)中,ROP6编码蛋白以GTP依赖的方式与Nod因子受体5(nodule factor receptor 5,NFR5)结合参与根毛发育,诱导根毛变形[8]。总之,ROP2/4/6在成毛细胞形成之前受YIP4a/b的激活,YIP4a/b影响ROP2/4/6的质膜积累;类固醇分子RHP1作用于Rop引导的局部信号通路并参与根毛尖端生长。
Rop鸟嘌呤核苷酸交换因子(rop-guanine nucleotide exchange factors,RopGEF)也参与根毛起始。RopGEF激活并催化Rop从不活跃的GDP结合状态到活跃的GTP结合状态的转变[9-10],调控Rop参与的细胞过程,如肌动蛋白组装、囊泡运输等。RopGEF3定位于根毛起始部位的质膜,主要调控根毛的起始和隆起。在拟南芥中,RopGEF3的T-DNA插入突变体植株根毛起始延迟,根毛密度减少;过表达RopGEF3诱导ROP2蛋白的积累,同时诱导Rop募集结构域(Rop-recruiting domain)异位形成,是细胞极化过程中产生根毛位置信号的标志点[11]。在水稻中,RopGEF3功能缺失的突变体表现为根毛变短且直径增加[12];RopGEF4功能缺失的突变体根毛长度减少;RopGEF10在根表皮细胞中表达,其功能缺失突变体表现为根毛密度减少[13]。表明RopGEF调控根毛尖端生长、根毛直径及根毛密度,激活并参与Rop调控的细胞过程。
植物快速碱化因子(rapid alkalinization factors,RALF1)是一种分泌肽,与属于类受体激酶的FER(FERONIA)结合,调节植物细胞的扩张,促进ROP2和基本螺旋―环―螺旋(basic helix-loop- helix,bHLH)转录因子RSL4的mRNA翻译,促进根毛发育。在T-DNA插入系突变体fer植株中ROP的活性降低,FER是ROP信号通路的上游调控因子。FER、RopGEF和ROP在体外形成蛋白复合物来调控根毛发育[12⇓-14]。胞外信号激活FER后,RopGEF激活ROP2,活化的ROP2在根毛起始位点聚集,并激活下游定位于质膜的RHD2效应因子,RHD2与尖端Ca2+浓度梯度共同调节根毛生长[14]。拟南芥AGC1.5(cAMP-dependent protein kinase A cGMP-dependent protein kinase G phospholipid-dependent protein kinase C)亚家族激酶与ROP的2个效应蛋白ICR2(interactor of constitutive active ROP 2)或MIDD1(microtubule depletion domain1)、ROP三者形成复合体,通过将AGC1.5激酶招募到质膜上活性ROP的区域,并促进AGC1.5对于Rop GEF的磷酸化,保证Rop GEF在根毛生长过程中的尖端定位,维持根毛生长过程中ROP信号的极性动态分布,对于根毛尖端生长的建立和维持至关重要[15-16]。
综上,Rop GEF激活ROP2,ROP2决定根毛起始并促进根毛尖端生长。类固醇分子RHP1作用于Rop引导的局部信号通路,调控根毛尖端生长,同样参与RHD6-RSL4形成的调控途径,也参与调控根毛发育,类受体激酶FER、Rop和Rop GEF形成的蛋白复合体共同调节根毛发育。
2 转录因子调控途径
涉及根毛发育过程的转录因子包括bHLH、GTL1(GT-2-LIKE1)、GL2(GLABRA2)、GL3(GLABRA3)和EGL3(ENHANCEROFGLABRA3)等。在拟南芥中,根毛的起始和生长受到RHD6、RSL1(RHD6-LIKE1)、RSL2、RSL3、RSL4、RHL1(ROOTHAIRLESS1)、LRL1(LJ-RHL1-LIKE1)、LRL2和LRL5等bHLH转录因子调控。bHLH在拟南芥调控根表皮细胞形成的基因下游起作用。RHD6和RSL1编码bHLH VIIIc亚家族的转录因子,决定根毛起始,是根毛细胞发育所必需的调控基因[17⇓-19]。拟南芥中,RHD6正向调节成毛细胞的发育,rhd6突变体只产生少量根毛,RHD6同源基因RSL1功能缺失突变体rsl1的根毛产生不受影响,而rhd6/rsl1双突变体则不产生根毛,表明RHD6和RSL1在根毛发育中功能部分冗余[18]。RHD6和RSL1能调控RSL4在根毛中的表达,也能调控RSL2、RSL3和LRL3等参与根毛的生长[19⇓-21]。拟南芥Ⅰ类RSL基因正向调节Ⅱ类RSL基因RSL2、RSL3、RSL4和RSL5的表达和根毛发育,拟南芥Ⅰ类RSL基因由于同源异型结构域转录抑制因子GL2的存在不足以进行根毛发育。水稻Ⅰ类RSL基因RSL1、RSL2和RSL3正向调节水稻根毛发育,异位过表达会导致异位根毛细胞发育,异位表达后水稻表皮大部分细胞转化为根毛细胞,表明拟南芥和水稻抑制RSL Ⅰ类基因活性的机制存在差异[18,22-23]。
bHLH转录因子RSL4是根毛发育过程中响应胞内外信号、调节根毛细胞大小的关键因子。在根毛形成前,RSL4在表皮细胞核中逐渐积累达到最大值;在根毛生长过程中,RSL4的积累逐渐降低,随着RSL4减少根毛停止生长,表明RSL4的积累直接决定了根毛细胞的生长[24]。在根毛形态建成中起作用的根毛特异性基因RHS(ROOT HAIR SPECIFIC)其调控区域包含根毛特异性顺式元件RHE(RHS CIS-ELEMENT),RSL4能与RHE结合上调RHS,促进根毛发育[25-26]。含有植物特异性三螺旋转录因子GT-2家族的GTL1通过抑制细胞周期,调控基因的表达来终止细胞生长,其功能缺失突变体gtl1产生较大的毛状体。RSL4能上调呼吸爆发氧化酶同系物(respiratory burst oxidase homologoues,RBOH)和过氧化物酶(peroxiredoxin,PRX)的表达,并且受GTL1调控抑制不必要的根毛形成,以此维持根毛的生长平衡[23,27-28]。RHL1编码根毛发育所需的XIII类bHLH转录因子。在水稻中,RHL1突变体的根长、侧根和不定根数量无明显表型,根毛直径不变、长度减少;RHL1过表达会导致根毛变长[29],表明RHL1在根毛长度变化中起正向调控作用。在玉米中,LRL5可能直接参与协调根毛生长过程中的蛋白翻译过程和核糖体基因的表达,并调控根毛尖端生长,LRL5敲除突变体产生短根毛,根毛数量不变;LRL5表达量增加抑制根毛生长相关蛋白的翻译,导致根毛生长率降低[30]。综上,bHLH转录因子RSL4受到GTL1的抑制,RSL4和RHE结合上调RHS促进根毛发育,也可通过上调RBOH和PRX的表达来维持根毛生长平衡。同样,bHLH转录因子RHL1正向调控根毛长度变化,LRL5调节根毛蛋白翻译和核糖体基因的表达。
拟南芥毛状体发育主要受核心毛状体起始调控因子MBW(MYB-bHLH-WD40)转录复合物及毛状体调控因子GL2控制[31-32]。MBW转录复合物由R2R3型MYB转录因子WER(WERWOLF)、bHLH型转录因子和WD40[WER-GL3-TTG1(TRANSPARENT TESTA GLABRA1)]重复蛋白组成,该转录复合物激活GL2的表达,随后GL2抑制RHD6以及RSL4等一系列下游转录因子及其靶基因来阻断根毛的起始发育[33-34]。GL2属于拟南芥亮氨酸拉链蛋白(homeodomain-leucine zipper,HD-Zip)家族转录因子,是调控表皮毛形成和发育的关键因子[35]。在拟南芥中,GL2调控C2H2型锌指蛋白ZP1(ZINC FINGER PROTEIN1)的表达,抑制RHD6、RSL4和RSL2的转录,负调控根毛的起始和生长[36]。GL2启动子也能调控bHLH转录因子LRL1和LRL2的表达,促进N型细胞的发育[22]。在拟南芥中,编码bHLH蛋白的GL3与EGL3部分功能冗余以决定根表皮细胞命运,MYC1(MYC PROTO-ONCOGENE,BHLH TRANSCRIPTION FACTOR)与GL3、EGL3属于同一亚族,MYC1也在根表皮细胞命运决定中发挥作用,并促进GL2表达[19,37]。GL3表达也受到含有Jmj-C(Jumonji-C)结构域的组蛋白去甲基酶JMJ29(Jumonji29)的直接调节,参与毛状体发育[38]。综上,GL2决定根表皮毛细胞命运调控表皮毛形成和发育,GL2通过GL2-ZP1-RHD6途径负调控根毛起始和生长,GL3、EGL3通过JMJ29-GL3-bHLH途径调节毛状体发育。
由此可见,bHLH转录因子在根毛细胞命运决定、根毛起始及根毛生长发育中发挥重要作用。核心毛状体起始调控因子MBW及毛状体调控因子GL2调控根毛起始,RHD6、RSL1及RSL4形成的转录调控途径调节根毛发育。
3 Ca2+调控途径
负责编码根毛尖端生长所需Ca2+信号的环状核苷酸门控阳离子通道基因CNGC(cyclic nucleotide gated cation channels)是调控胞质信号分子的离子通道,其是植物细胞中Ca2+流动的主要通道[42]。拟南芥CNGC1主要在幼根中表达,该基因表达能增强拟南芥幼苗对Ca2+的吸收,CNGC1缺失突变导致幼根中Ca2+含量略低于野生型(6%~ 22%),初生根生长速率增加[43]。CNGC5、CNGC6、CNGC9和CNGC14[25]是根毛尖端Ca2+振荡的中央调节因子,参与调控根毛的生长,这4种CNGC基因在产生和维持根毛尖端Ca2+振荡上功能冗余。Brost等[44]研究表明,cngc6/9/14三突呈现Ca2+振荡受影响,根毛快速生长后一段时间根毛细胞破裂,不能形成正常的根毛。cngc5/6/9三突根毛显著缩短并产生分枝,此根毛生长缺陷可以通过表达CNGC5、CNGC6、CNGC9或外施高浓度Ca2+挽救[39,41,44]。CNGC14突变体T-DNA插入系(CNGC14-1)和microRNA173的RNA干扰转基因系(cngc14-miRNA173)产生短根毛表型[45]。钙调蛋白7(Calmodulin7,CaM7)为Ca2+传感器,CaM7与CNGC14直接相互作用,CaM7的第3个EF-hand结构域与CNGC14胞质C端结构域特异性相互作用,CaM7抑制CNGC14介导的Ca2+内流调控根毛的极性生长[44⇓-46]。综上,CNGC是Ca2+流动和植株吸收Ca2+的主要离子通道,CNGC突变体根毛尖端正常Ca2+振荡受影响,不能形成正常根毛,表现为短根毛或分枝表型。CNGC、Ca2+及CaM7协调控制根毛极性生长和生长方向。
Bai 等[47]发现类受体激酶Ca2+细胞相关蛋白激酶1([Ca2+]cyt-associated protein kinase,CAP1)通过维持[Ca2+]cyt梯度来调节根毛尖端生长。在拟南芥中,CAP1响应NH4+浓度调控根毛生长,CAP1基因敲除突变体cap1-1中细胞质NH4+水平升高,并且cap1-1在NH4+胁迫条件下,根系产生短根毛[47-48],GEF1为CAP1的相互作用因子,cap1缺失产生短根毛,GEF1过表达一定程度上缓解cap1短根毛的产生[49]。在芥菜(Brassica juncea L.)中,植物镉抗性(plant cadmium resistance,PCR)家族蛋白BjPCR1是Ca2+从根表皮转移到内细胞并最终转移到地上部所需的外排转运蛋白,参与根中Ca2+高效转运。在拟南芥中根毛特异性表达BjPCR1植株的Ca2+抗性和转运速率增强[50]。植物感知细菌Nod因子或真菌信号,诱导根毛细胞核内Ca2+振荡,进而激活钙调蛋白(CaM)和Ca2+/CaM依赖性蛋白激酶(Ca2+/CaM-dependent protein kinase,CCaMK),CCaMK与Ca2+、CaM两者结合后磷酸化转录因子启动下游信号事件[51]。RHD2还原烟酰胺腺嘌呤二核苷酸磷酸(NADPH)氧化酶产生的ROS,刺激拟南芥根毛生长所需的Ca2+内流到细胞质中,RBOH产生ROS作为重要的第二信使启动Ca2+通道,在根毛尖端形成Ca2+浓度梯度,从而调控微丝的动态变化和囊泡运输来调控根毛的产生[14,25,52]。RSL4与根毛生长抑制因子3(repressor of excessive roothair growth3,RXR3)启动子内的RHE结合,RXR3受RSL4转录激活,在Ca2+存在条件下,CaM7在RXR3上游起作用,影响根毛生长和顶端[Ca2+]cyt振荡,在RXR3突变体rxr3中[Ca2+]cyt振荡的振幅受抑制[53]。综上,[Ca2+]cyt受到类受体激酶、PCR家族蛋白、Nod因子的调控,促使Ca2+在根中的高效转运。呼吸爆发氧化酶同系物RBOH产生的ROS启动Ca2+通道,协调根中Ca2+平衡,调节根毛尖端生长及根毛延伸。RXR3受RHL4的转录激活,CaM7在RXR3上游起作用,影响根毛生长和顶端[Ca2+]cyt振荡,调控根毛尖端生长。
4 磷酸盐调控途径
磷(P)是最重要的常量营养元素之一,是核酸、膜磷脂和能量代谢物的结构成分。植物从土壤中吸收无机磷酸盐(Pi),Pi缺乏是限制植物生长和产量的主要因素之一[54]。植物的根系利用适应机制在土壤中吸收Pi形式的磷[55⇓-57],根毛是植物吸收磷的主要部位,根毛增加和根表面积对养分吸收率的增强被认为是缺磷条件下典型的根系形态响应。拟南芥中,正常条件下根毛较少或较短的自交系在低浓度磷条件(1 μmol/L)下根毛密度增加,相反根毛浓密或较长的自交系根毛密度更低或长度更短[58]。缺磷(0 μmol/L)处理条件下,水稻根毛长度和密度均显著增加[59],拟南芥初生根生长受到抑制,植株通过增加侧根和根毛的发生来适应Pi缺乏[60]。
拟南芥中,低磷可诱导生长素的合成、运输和信号传导,低磷条件下的乙烯突变体ein(eihylene inseneitive)根毛长度明显减少。低磷条件诱导生长素响应因子19(auxin response factor19,ARF19)的表达,并上调转录因子RSL2和RSL4的表达来促进根毛的伸长[61-62]。磷浓度较高(5 mmol/L)能够强烈抑制与极性生长下降相关的RSL4和RSL2的表达[63]。拟南芥中,MYB30和乙烯转录因子EIN3通过对RSL4和其他磷饥饿响应基因PSR(PI STARVATION-RESPONSIVE)的转录微调低磷条件下磷吸收,调节根毛生长[54]。磷饥饿增加了EIN3蛋白的水平,EIN3蛋白结合到RSL4和RSL4的同源基因调控的基因的启动子上,进一步增加其转录进程,促进根毛数量增加[57]。在拟南芥中,磷胁迫(3 μmol/L)诱导RXR1编码过量磷响应蛋白DUF506(domain of unknown function 506 proteins),RXR1与RabD2c GTPase(Ras-associated binding D2c GTPase)相互作用抑制根毛生长[64]。拟南芥中,磷脂酰肌醇4,5-二磷酸(PtdIns(4,5)P2)与质膜相关Ca2+结合蛋白2(PCaP2)竞争性结合负调控根毛尖端生长并减弱根毛伸长,pcap2敲除系根毛更长[65-66]。综上,MYB30、EIN3、RSL4及磷响应蛋白在不同浓度磷酸盐条件下影响根毛对磷的吸收,协调根毛生长发育,磷与Ca2+结合蛋白作用调控根毛尖端生长,影响根毛长度。
5 生长素调控途径
生长素是一种重要的植物激素,主要在根尖、茎尖及发育中的叶原基中合成。生长素作为环境和激素途径的组织节点调控根毛生长[67],在根毛细胞命运的决定、根毛形成及根毛生长中起调控作用。rhd6突变体根毛密度减少,长度变短,生长素(吲哚乙酸)处理能恢复rhd6的根毛密度和长度。rhd6-3/rsl1-1双突植株不产生根毛,生长素处理能恢复rhd6-3/rsl1-1植株的根毛生长,表明生长素在根毛发育过程中作用于RHD6和RSL1的下游调控途径。生长素处理提高RSL4转录表达水平和RSL4蛋白水平,表明生长素诱导根中RSL4的表达,调控根毛的生长,且RSL4蛋白活性是生长素刺激根毛生长所必需的[20,68-69]。生长素不敏感突变体axr2(auxin resistant2)和乙烯响应突变体etr1(ethylene response1)只有少数根毛发生,且主要位于细胞的基部(远离根分生组织),这表明生长素和乙烯既参与调控根毛起始数量,又调控根毛在细胞中隆起位置[69-70]。在根毛生长过程中,生长素从根尖极性运输到新生根毛细胞,这一过程由生长素载体介导。AUX1/LAX(AUXIN1/LIKE- AUX1)是生长素输入载体[71],参与不同器官和组织中生长素相关的发育过程。拟南芥AUX1基因仅在侧根冠和表皮细胞层表达,AUX1和LAX3均与侧根的发育和根尖的形成有关,调控根的向地性和根毛发育[72]。TIR1/ AFB(TRANSPORT INHIBITOR RESISTANT1/ AUXIN SIGNALING F-BOX)是SCF(SKP/ CULLIN1/F-Box)E3泛素连接酶底物识别亚单元。生长素与TIR1/AFB结合,促进TIR1/AFB与AUX/ IAA互作并泛素化AUX/IAA[73]。AUX1转运体、生长素受体SCFTIR1/AFB和CNGC14 Ca2+通道介导根系中的生长素信号传导[74]。GNOM是ADP-核糖基化因子(ADP-ribosylation factor,Arf)G蛋白上的一种膜相关的GEF。AUX1[75-76]、EIN2(ETHYLENE INSENSITIVE2)[77]和GNOM基因[78]共同作用调控根毛的发生位置[79]。在水稻中,AUX1突变体根毛长度增加[80],AUX3基因的2个独立敲除系AUX3-1和AUX3-2具有较短主根、较低侧根密度和较长根毛[81]。
PIN和PGP/ABCB(PGLYCOPROTEIN/ATP- BINDING CASSETTEB4)是生长素输出载体[82-83]。生长素输出载体PIN2主要定位于幼嫩皮层细胞的基膜,也定位于表皮、侧根冠和上皮层细胞的根尖胞质膜[84],在根尖生长素浓度梯度建立方面起着重要的作用[85]。根毛细胞中6个PIN(PIN1~PIN4、PIN7和PIN8)的过表达抑制了根毛的生长,可能是由于生长素输出降低了根毛细胞中的生长素水平[86]。PIN5主要定位于内质网,其在根毛细胞中的表达促进了根毛生长,增强了内源性生长素的作用[86]。PIN5、PIN6、PIN8和PILS(PIN-LIKES)生长素转运蛋白参与细胞内生长素再分配[87]。PIN6在植物发育过程中调节生长素的水平,pin6功能丧失突变体影响初生根的生长和侧根的发育,PIN6的表达缺失影响根毛生长和根波动[88],表明PIN对根毛的生长既有促进作用又有抑制作用。
综上,生长素可促进根毛生长,AUX1/LAX作为生长素输入载体影响生长素相关的根毛发育过程,PIN和PGP/ABCB作为生长素输出载体,其不同的表达水平影响根毛的生长发育。总之,载体介导的生长素运输途径对于植物根毛生长至关重要。
6 展望
根毛极性生长受Rop介导的信号途径和RHD6、RSL1-RSL4介导的转录激活途径共同调控,RSL4是RHD6的直接转录靶点,RHD6、RSL1能调控RSL4的表达,然而这2条途径之间的联系仍然不清楚[7]。推测Rop信号途径可能和RHD6-RSL4途径协同工作来支持根毛的持续极性生长。本文ROP2决定根毛起始,Rop引导的局部信号通路作用于RHD6-RSL4依赖的转录通路上游,形成的调控途径影响根毛尖端生长发育,Rop与类受体激酶、Rop GEF等相互作用调节根毛起始和尖端生长。由此可见,根毛的发育是个复杂的网络,与Rop调控途径、激活Rop的Rop GEF以及促进Rop转录的类受体激酶FER等途径和调控基因紧密相关,也与GL2-RHD6-RSL4调控途径、bHLH家族转录因子RSL1、RSL4、RHD6、RHL1等转录因子相关。
CNGC传导Ca2+形成相应离子通道调节根毛生长。Ca2+信号传导主要分为编码(细胞在外界刺激的触发下诱导Ca2+的流动)、解码(被传感器蛋白识别)和响应(调控下游细胞反应)3个步骤。编码过程需要一系列复杂的通道和转运蛋白,但解码过程具有许多Ca2+传感器和效应器,可将Ca2+信号转换为细胞效应,根毛尖端聚集的Ca2+指定了细胞快速生长的信号事件。CaM7是RXR3抑制根毛生长的必要条件,磷限制显著诱导了RXR3的转录水平和蛋白水平,但磷限制诱导的RXR3蛋白积累是否影响CaM-CNGC14的相互作用仍需进一步研究。Koster等[89]对植物免疫中的Ca2+信号进行综述,尽管已经鉴定出许多候选通道蛋白促进模式触发免疫或效应物触发免疫,但关于通道早期Ca2+信号瞬变的机制仍然未知。植物胞内免疫受体NLR(nucleotide-binding leucine-rich repeat,NB-LRR)蛋白可作为Ca2+通道发挥作用,介导细胞死亡和防御反应[90]。Ca2+还在植物生长发育和各种逆境胁迫响应中发挥重要作用。最近Zhang等[91]研究发现,镉胁迫激发植物内Ca2+信号,揭示植物通过质膜Ca2+信号来调控镉胁迫。由此可见,Ca2+信号在植物中的应用很广泛,未来对Ca2+的研究将包括免疫Ca2+信号通道及传导、其他物质通过Ca2+通道对植物的协同调控等方面。
参考文献
Clonal relationships and cell patterning in the root epidermis of Arabidopsis
Co-regulation of root hair tip growth by ROP GTPases and nitrogen source modulated pH fluctuations
Extracellular signals and receptor-like kinases regulating ROP GTPases in plants
DOI:10.3389/fpls.2014.00449
PMID:25295042
[本文引用: 1]

Rho-like GTPase from plants (ROPs) function as signaling switches that control a wide variety of cellular functions and behaviors including cell morphogenesis, cell division and cell differentiation. The Arabidopsis thaliana genome encodes 11 ROPs that form a distinct single subfamily contrarily to animal or fungal counterparts where multiple subfamilies of Rho GTPases exist. Since Rho proteins bind to their downstream effector proteins only in their GTP-bound "active" state, the activation of ROPs by upstream factor(s) is a critical step in the regulation of ROP signaling. Therefore, it is critical to examine the input signals that lead to the activation of ROPs. Recent findings showed that the plant hormone auxin is an important signal for the activation of ROPs during pavement cell morphogenesis as well as for other developmental processes. In contrast to auxin, another plant hormone, abscisic acid, negatively regulates ROP signaling. Calcium is another emerging signal in the regulation of ROP signaling. Several lines of evidence indicate that plasma membrane localized-receptor like kinases play a critical role in the transmission of the extracellular signals to intracellular ROP signaling pathways. This review focuses on how these signals impinge upon various direct regulators of ROPs to modulate various plant processes.
Rho-of-plant activated root hair formation requires Arabidopsis YIP4a/b gene function
The Arabidopsis Rop 2 GTPase is a positive regulator of both root hair initiation and tip growth
Small molecule RHP 1 promotes root hair tip growth by acting upstream of the RHD6-RSL4- dependent transcriptional pathway and ROP signaling in plants
ROP6 is involved in root hair deformation induced by Nod factors in Lotus japonicus
DOI:S0981-9428(16)30340-0
PMID:27592173
[本文引用: 1]

Roots of leguminous plants perceive Nod factor signals, and then root hair deformation responses such as swelling and curling are activated. However, very little is known about the molecular mechanisms of such root hair deformation. We have previously shown that LjROP6, a member of the Rho family of small GTPases, was identified as an NFR5 (Nod Factor Receptor 5)-interacting protein and participated in symbiotic nodulation in Lotus japonicus. In this study, we identified ten LjROP GTPases including LjROP6, and they were distributed into groups II, III, IV but not group I by phylogenetic analysis. The expression profiles of ten LjROP genes during nodulation were examined. LjROP6 belonged to group IV and interacted with NFR5 in a GTP-dependent manner. Overexpression of either wild-type ROP6 or a constitutively active mutant (ROP6-CA) generated root hair tip growth depolarization, while overexpression of a dominant negative mutant (ROP6-DN) exhibited normal root hair growth. After inoculating with Mesorhizobium loti or adding Nod factors to hairy roots, overexpression of ROP6 and ROP6-CA exhibited extensive root hair deformation, while overexpression of ROP6-DN inhibited root hair deformation. The infection event and nodule number were increased in ROP6 and ROP6-CA overexpressing transgenic plants; but decreased in ROP6-DN overexpressing transgenic plants. These studies provide strong evidence that ROP6 GTPase, which binds NFR5 in a GTP-dependent manner, is involved in root hair development as well as root hair deformation responses induced by NFs in the early stage of symbiotic interaction in L. japonicus.Copyright © 2016 Elsevier Masson SAS. All rights reserved.
A new family of RhoGEFs activates the Rop molecular switch in plants
Members of a novel class of Arabidopsis Rho guanine nucleotide exchange factors control rho GTPase-dependent polar growth
Distinct RopGEFs successively drive polarization and outgrowth of root hairs
DOI:S0960-9822(19)30488-9
PMID:31104938
[本文引用: 1]

Root hairs are tubular protrusions of the root epidermis that significantly enlarge the exploitable soil volume in the rhizosphere. Trichoblasts, the cell type responsible for root hair formation, switch from cell elongation to tip growth through polarization of the growth machinery to a predefined root hair initiation domain (RHID) at the plasma membrane. The emergence of this polar domain resembles the establishment of cell polarity in other eukaryotic systems [1-3]. Rho-type GTPases of plants (ROPs) are among the first molecular determinants of the RHID [4, 5], and later play a central role in polar growth [6]. Numerous studies have elucidated mechanisms that position the RHID in the cell [7-9] or regulate ROP activity [10-18]. The molecular players that target ROPs to the RHID and initiate outgrowth, however, have not been identified. We dissected the timing of the growth machinery assembly in polarizing hair cells and found that positioning of molecular players and outgrowth are temporally separate processes that are each controlled by specific ROP guanine nucleotide exchange factors (GEFs). A functional analysis of trichoblast-specific GEFs revealed GEF3 to be required for normal ROP polarization and thus efficient root hair emergence, whereas GEF4 predominantly regulates subsequent tip growth. Ectopic expression of GEF3 induced the formation of spatially confined, ROP-recruiting domains in other cell types, demonstrating the role of GEF3 to serve as a membrane landmark during cell polarization.Copyright © 2019 Elsevier Ltd. All rights reserved.
Interaction of OsRopGEF 3 protein with OsRac3 to regulate root hair elongation and reactive oxygen species formation in rice (Oryza sativa)
Arabidopsis RopGEF4 and RopGEF 10 are important for FERONIA-mediated developmental but not environmental regulation of root hair growth
Local positive feedback regulation determines cell shape in root hair cells
DOI:10.1126/science.1152505
PMID:18309082
[本文引用: 3]

The specification and maintenance of growth sites are tightly regulated during cell morphogenesis in all organisms. ROOT HAIR DEFECTIVE 2 reduced nicotinamide adenine dinucleotide phosphate (RHD2 NADPH) oxidase-derived reactive oxygen species (ROS) stimulate a Ca2+ influx into the cytoplasm that is required for root hair growth in Arabidopsis thaliana. We found that Ca2+, in turn, activated the RHD2 NADPH oxidase to produce ROS at the growing point in the root hair. Together, these components could establish a means of positive feedback regulation that maintains an active growth site in expanding root hair cells. Because the location and stability of growth sites predict the ultimate form of a plant cell, our findings demonstrate how a positive feedback mechanism involving RHD2, ROS, and Ca2+ can determine cell shape.
A positive feedback circuit for ROP-mediated polar growth
DOI:10.1016/j.molp.2020.11.017
PMID:33271334
[本文引用: 1]

Tip growth is a special type of polarized growth in which a single and unique polarization site is established and maintained. Rho of Plants (ROP) proteins, which represent the only class of Rho GTPases in plants, regulate tip growth. The dynamic and asymmetric distribution of ROPs is critical for the establishment and maintenance of tip growth, and requires at least one positive feedback loop, which is still elusive. Here, we report a positive feedback circuit essential for tip growth of root hairs, in which ROPs, ROP activators and effectors, and AGC1.5 subfamily kinases are interconnected by sequential oligomerization and phosphorylation. AGC1.5 subfamily kinases interact with and phosphorylate two guanine nucleotide exchange factors (GEFs) of ROPs, RopGEF4 and RopGEF10. They also interact with two ROP effectors, ICR2/RIP3 and MIDD1/RIP4, which bridge active ROPs with AGC1.5. Functional loss of the AGC1.5 subfamily kinases or ICR2 and MIDD1 compromised root hair growth due to reduced ROP signaling. We found that asymmetric targeting of RopGEF4 and RopGEF10 is controlled by AGC1.5-dependent phosphorylation. Interestingly, we discovered that the ROP effectors recruit AGC1.5 to active ROP domains at the plasma membrane during root hair growth and are critical for AGC1.5-dependent phosphorylation of RopGEFs. Given the large number of AGC kinases in plants, this positive feedback circuit may be a universal theme for plant cell polar growth.Copyright © 2020 The Author. Published by Elsevier Inc. All rights reserved.
AGC1.5 kinase phosphorylates RopGEFs to control pollen tube growth
DOI:S1674-2052(18)30222-3
PMID:30055264
[本文引用: 1]

Double fertilization in angiosperms requires the targeted delivery of immotile sperm to the eggs through pollen tubes. The polarity of tip-growing pollen tubes is maintained through dynamic association of active Rho GTPases of plants (ROP-GTP) with the apical plasma membrane. Guanine nucleotide exchange factors for ROPs (RopGEFs) catalyze the activation of ROPs and thereby affect spatiotemporal ROP signaling. Whereas RopGEFs have been found to be phosphorylated proteins, the kinases responsible for their phosphorylation in vivo and biological consequences of RopGEF phosphorylation in pollen tube growth remain unclear. We report here that the Arabidopsis AGC1.5 subfamily of cytoplasmic kinases is critical for the restricted localization of ROP-GTP during pollen tube growth. Loss of AGC1.5 and AGC1.7 functions resulted in the mistargeting of active ROPs and defective events downstream of ROP signaling in pollen tubes. AGC1.5 interacts with RopGEFs via their catalytic PRONE domain and phosphorylates RopGEFs at a conserved Ser residue of PRONE domain. Loss of AGC1.5 and AGC1.7 functions resulted in the mistargeting of RopGEFs in pollen tubes, similar to the phenotype caused by the mutation that renders RopGEFs non-phosphorylatable by AGC1.5. Collectively, our results provide mechanistic insights into the spatiotemporal activation of ROPs during the polar growth of pollen tubes.Copyright © 2018 The Author. Published by Elsevier Inc. All rights reserved.
The basic helix-loop- helix transcription factor family in plants: a genome-wide study of protein structure and functional diversity
An ancient mechanism controls the development of cells with a rooting function in land plants
DOI:10.1126/science.1142618
PMID:17556585
[本文引用: 3]

Root hairs and rhizoids are cells with rooting functions in land plants. We describe two basic helix-loop-helix transcription factors that control root hair development in the sporophyte (2n) of the angiosperm Arabidopsis thaliana and rhizoid development in the gametophytes (n) of the bryophyte Physcomitrella patens. The phylogeny of land plants supports the hypothesis that early land plants were bryophyte-like and possessed a dominant gametophyte and later the sporophyte rose to dominance. If this hypothesis is correct, our data suggest that the increase in morphological complexity of the sporophyte body in the Paleozoic resulted at least in part from the recruitment of regulatory genes from gametophyte to sporophyte.
A gene regulatory network for root epidermis cell differentiation in Arabidopsis
A basic helix-loop-helix transcription factor controls cell growth and size in root hairs
DOI:10.1038/ng.529
PMID:20139979
[本文引用: 2]

Postmitotic cell growth defines cell shape and size during development. However, the mechanisms regulating postmitotic cell growth in plants remain unknown. Here we report the discovery of a basic helix-loop-helix (bHLH) transcription factor called RSL4 (ROOT HAIR DEFECTIVE 6-LIKE 4) that is sufficient to promote postmitotic cell growth in Arabidopsis thaliana root-hair cells. Loss of RSL4 function resulted in the development of very short root hairs. In contrast, constitutive RSL4 expression programmed constitutive growth, resulting in the formation of very long root hairs. Hair-cell growth signals, such as auxin and low phosphate availability, modulate hair cell extension by regulating RSL4 transcript and protein levels. RSL4 is thus a regulator of growth that integrates endogenous developmental and exogenous environmental signals that together control postmitotic growth in root hairs. The control of postmitotic growth by transcription factors may represent a general mechanism for regulating cell size across diverse organisms.
Recruitment and remodeling of an ancient gene regulatory network during land plant evolution
Proceedings of the National Academy of Sciences of the United States of America,
RSL class I genes positively regulate root hair development in Oryza sativa
GTL1 and DF 1 regulate root hair growth through transcriptional repression of ROOT HAIR DEFECTIVE 6-LIKE 4 in Arabidopsis
Intensity of a pulse of RSL4 transcription factor synthesis determines Arabidopsis root hair cell size
Tracheophytes contain conserved orthologs of a basic helix-loop-helix transcription factor that modulate ROOT HAIR SPECIFIC genes
ROS and calcium oscillations are required for polarized root hair growth
Trihelix transcription factors GTL1 and DF 1 prevent aberrant root hair formation in an excess nutrient condition
The Trihelix transcription factor GT2-like 1 (GTL1) promotes salicylic acid metabolism,and regulates bacterial-triggered immunity
A transcription factor with a bHLH domain regulates root hair development in rice
DOI:10.1038/cr.2009.109 PMID:19752888 [本文引用: 1]
Characterization of LRL5 as a key regulator of root hair growth in maize
EIN3 and RSL4 interfere with an MYB-bHLH-WD40 complex to mediate ethylene-induced ectopic root hair formation in Arabidopsis
Proceedings of the National Academy of Sciences of the United States of America.
The HD-ZIP IV transcription factor GL2-LIKE regulates male flowering time and fertility in cucumber
DOI:10.1093/jxb/eraa251
PMID:32490515
[本文引用: 1]

Cucumber is dioecious by nature, having both male and female flowers, and is a model system for unisexual flower development. Knowledge related to male flowering is limited, but it is reported to be regulated by transcription factors and hormone signals. Here, we report functional characterization of the cucumber (Cucumis sativus) GL2-LIKE gene, which encodes a homeodomain leucine zipper (HD-ZIP) IV transcription factor that plays an important role in regulating male flower development. Spatial-temporal expression analyses revealed high-level expression of CsGL2-LIKE in the male flower buds and anthers. CsGL2-LIKE is closely related to AtGL2, which is known to play a key role in trichome development. However, ectopic expression of CsGL2-LIKE in Arabidopsis gl2-8 mutant was unable to rescue the gl2-8 phenotype. Interestingly, the silencing of CsGL2-LIKE delayed male flowering by inhibiting the expression of the florigen gene FT and reduced pollen vigor and seed viability. Protein-protein interaction assays showed that CsGL2-LIKE interacts with the jasmonate ZIM domain protein CsJAZ1 to form a HD-ZIP IV-CsJAZ1 complex. Collectively, our study indicates that CsGL2-LIKE regulates male flowering in cucumber, and reveals a novel function of a HD-ZIP IV transcription factor in regulating male flower development of cucumber.© Society for Experimental Biology 2020.
MYB-bHLH-WD40 protein complex and the evolution of cellular diversity
DOI:10.1016/j.tplants.2004.12.011
PMID:15708343
[本文引用: 1]

A protein complex composed of MYB and bHLH transcription factors associated with a WD40 repeat protein initiates multiple cellular differentiation pathways in a range of plants. Recent reports have provided the first coherent models of the network of interactions that lead to diverse cell fates through the activity of this protein complex. The resulting flexibility in plant morphology is likely to have played a major role in angiosperm evolution and success. The complex appears to have arisen in the land plant lineage, although its component parts are considerably more ancient. Here, we review the evolutionary history of the MYB-bHLH-WD40 protein complex and its role in generating plant epidermal cellular diversity.
A cell surface arabinogalactan-peptide influences root hair cell fate
DOI:10.1111/nph.16487
PMID:32064614
[本文引用: 1]

Root hairs (RHs) develop from specialized epidermal trichoblast cells, whereas epidermal cells that lack RHs are known as atrichoblasts. The mechanism controlling RH cell fate is only partially understood. RH cell fate is regulated by a transcription factor complex that promotes the expression of the homeodomain protein GLABRA 2 (GL2), which blocks RH development by inhibiting ROOT HAIR DEFECTIVE 6 (RHD6). Suppression of GL2 expression activates RHD6, a series of downstream TFs including ROOT HAIR DEFECTIVE 6 LIKE-4 (RSL4) and their target genes, and causes epidermal cells to develop into RHs. Brassinosteroids (BRs) influence RH cell fate. In the absence of BRs, phosphorylated BIN2 (a Type-II GSK3-like kinase) inhibits a protein complex that regulates GL2 expression. Perturbation of the arabinogalactan peptide (AGP21) in Arabidopsis thaliana triggers aberrant RH development, similar to that observed in plants with defective BR signaling. We reveal that an O-glycosylated AGP21 peptide, which is positively regulated by BZR1, a transcription factor activated by BR signaling, affects RH cell fate by altering GL2 expression in a BIN2-dependent manner. Changes in cell surface AGP disrupts BR responses and inhibits the downstream effect of BIN2 on the RH repressor GL2 in root epidermis.© 2020 The Authors. New Phytologist © 2020 New Phytologist Trust.
植物表皮毛发育控制基因GL2遗传互作因子的筛选和鉴定
DOI:10.13560/j.cnki.biotech.bull.1985.2016.11.019
[本文引用: 1]

表皮毛广泛存在于陆生植物的地上部分,是植物与环境之间的一道天然屏障,具有多种重要的生物学功能。拟南芥HD-Zip家族转录因子GLABRA 2(GL2)是调控表皮毛形成和发育的关键因子,通过筛选和鉴定GL2的遗传互作因子,可以为进一步研究植物表皮毛发育调控的分子机制奠定基础。通过大规模的遗传筛选和图位克隆,获得了一个叶片上完全没有表皮毛的突变体M12-01,遗传分析表明M12-01 single突变表型受隐性单核基因控制。M12-01 single突变体表型与拟南芥TRANSPARENT TESTA GLABKA 1(TTG1)基因的功能缺失突变体表型相似。对TTG1基因的测序结果显示其+445位碱基由鸟嘌呤突变为腺嘌呤,从而使编码的甘氨酸变为精氨酸。本研究证实TTG1突变能增强gl2-3突变体的表型,GL2基因与TTG1基因之间存在遗传互作,这为进一步研究GL2调控植物表皮毛发育的分子机制提供了新的遗传材料。
Arabidopsis ZINC FINGER PROTEIN1 acts downstream of GL 2 to repress root hair initiation and elongation by directly suppressing bHLH genes
The bHLH genes GLABRA 3 (GL3) and enhancer of GLABRA3 (EGL3) specify epidermal cell fate in the Arabidopsis root
Arabidopsis JMJ29 is involved in trichome development by regulating the core trichome initiation gene GLABRA3
Calcium spikes,waves and oscillations in plant development and biotic interactions
DOI:10.1038/s41477-020-0667-6
PMID:32601423
[本文引用: 2]

The calcium ion (Ca) is a universal signal in all eukaryotic cells. A fundamental question is how Ca, a simple cation, encodes complex information with high specificity. Extensive research has established a two-step process (encoding and decoding) that governs the specificity of Ca signals. While the encoding mechanism entails a complex array of channels and transporters, the decoding process features a number of Ca sensors and effectors that convert Ca signals into cellular effects. Along this general paradigm, some signalling components may be highly conserved, but others are divergent among different organisms. In plant cells, Ca participates in numerous signalling processes, and here we focus on the latest discoveries on Ca-encoding mechanisms in development and biotic interactions. In particular, we use examples such as polarized cell growth of pollen tube and root hair in which tip-focused Ca oscillations specify the signalling events for rapid cell elongation. In plant-microbe interactions, Ca spiking and oscillations hold the key to signalling specificity: while pathogens elicit cytoplasmic spiking, symbiotic microorganisms trigger nuclear Ca oscillations. Herbivore attacks or mechanical wounding can trigger Ca waves traveling a long distance to transmit and convert the local signal to a systemic defence program in the whole plant. What channels and transporters work together to carve out the spatial and temporal patterns of the Ca fluctuations? This question has remained enigmatic for decades until recent studies uncovered Ca channels that orchestrate specific Ca signatures in each of these processes. Future work will further expand the toolkit for Ca-encoding mechanisms and place Ca signalling steps into larger signalling networks.
Root hair growth in Arabidopsis thaliana is directed by calcium and an endogenous polarity
DOI:10.1007/s004250050219
PMID:9421933
[本文引用: 1]

Tip growth of plant cells has been suggested to be regulated by a tip-focused gradient in cytosolic calcium concentration ([Ca2+]c). However, whether this gradient orients apical growth or follows the driving force for this process remains unknown. Using localized photoactivation of the caged calcium ionophore Br-A23187 we have been able to artificially generate an asymmetrical calcium influx across the root hair tip. This led to a change in the direction of tip growth towards the high point of the new [Ca2+]c gradient. Such reorientation of growth was transient and there was a return to the original direction within 15 min. Root hairs forced to change the direction of their growth by placing a mechanical obstacle in their path stopped, reoriented growth to the side, and grew past the mechanical blockage. However, as soon as the growing tip had cleared the obstacle, growth returned to the original direction. Confocal ratio imaging revealed that a tip-focused [Ca2+]c gradient was always centered at the site of active growth. When the root hair changed direction the gradient also reoriented, and when growth returned to the original direction, so did the [Ca2+]c gradient. This normal direction of apical growth of Arabidopsis thaliana (L.) Heynh, root hairs was found to be at a fixed angle from the root of 85 +/- 6.7 degrees. In contrast, Tradescantia virginiana (L.) pollen tubes that were induced to reorient by touch or localized activation of the caged ionophore, did not return to the original growth direction, but continued to elongate in their new orientation. These results suggest that the tip-focused [Ca2+]c gradient is an important factor in localizing growth of the elongating root hair and pollen tube to the apex. However, it is not the primary determinant of the direction of elongation in root hairs, suggesting that other information from the root is acting to continuously reset the growth direction away from the root surface.
Three CNGC family members, CNGC5, CNGC6, and CNGC9, are required for constitutive growth of Arabidopsis root hairs as Ca2+-permeable channels
Calcium regulation of tip growth: new genes for old mechanisms
DOI:10.1016/j.pbi.2011.09.005
PMID:22000040
[本文引用: 1]

We review the recent advances on Ca(2+) in tip-growing cells, with a special focus on pollen tubes. New genes for Ca(2+) pumps, channels and sensing proteins have been recently described, with special emphasis on cyclic nucleotide gated channels (CNGCs) and glutamate receptor-like channels (GLRs). We also review the current state of knowledge in what concerns Ca(2+) sensor and relay proteins, where the knowledge of the cell models is less advanced. While these newly described genes offer promise to a better understanding of the spatial and temporal patterns of Ca(2+) signalling that may be relevant for the formation of the phenotype, we discuss the necessity to investigate further links in the network downstream of the Ca(2+) signature, with a special need for mechanisms of feed-back that might render functional feed-back loops approachable by modelling and genetics. Given the available literature, we conclude on the need to investigate more on the role of two specific classes of proteins, the calcium binding protein kinases (CPKs) and the Calcineurin B-like proteins (CBLs) and their regulatory relationships to ion channels (summarized in Figure 3b).Copyright © 2011 Elsevier Ltd. All rights reserved.
Characterization of plant phenotypes associated with loss-of-function of AtCNGC1, a plant cyclic nucleotide gated cation channel
Multiple cyclic nucleotide-gated channels coordinate calcium oscillations and polar growth of root hairs
DOI:10.1111/tpj.14371
PMID:31033043
[本文引用: 3]

Calcium gradients underlie polarization in eukaryotic cells. In plants, a tip-focused Ca -gradient is fundamental for rapid and unidirectional cell expansion during epidermal root hair development. Here we report that three members of the cyclic nucleotide-gated channel family are required to maintain cytosolic Ca oscillations and the normal growth of root hairs. CNGC6, CNGC9 and CNGC14 were expressed in root hairs, with CNGC9 displaying the highest root hair specificity. In individual channel mutants, morphological defects including root hair swelling and branching, as well as bursting, were observed. The developmental phenotypes were amplified in the three cngc double mutant combinations. Finally, cngc6/9/14 triple mutants only developed bulging trichoblasts and could not form normal root hair protrusions because they burst after the transition to the rapid growth phase. Prior to developmental defects, single and double mutants showed increasingly disturbed patterns of Ca oscillations. We conclude that CNGC6, CNGC9 and CNGC14 fulfill partially but not fully redundant functions in generating and maintaining tip-focused Ca oscillations, which are fundamental for proper root hair growth and polarity. Furthermore, the results suggest that these calmodulin-binding and Ca -permeable channels organize a robust tip-focused oscillatory calcium gradient, which is not essential for root hair initiation but is required to control the integrity of the root hair after the transition to the rapid growth phase. Our findings also show that root hairs possess a large ability to compensate calcium-signaling defects, and add new players to the regulatory network, which coordinates cell wall properties and cell expansion during polar root hair growth.© 2019 The Authors The Plant Journal © 2019 John Wiley & Sons Ltd.
Arabidopsis CNGC14 mediates calcium influx required for tip growth in root hairs
DOI:S1674-2052(17)30067-9 PMID:28286297 [本文引用: 2]
The interaction of CaM7 and CNGC 14 regulates root hair growth in Arabidopsis
A receptor-like kinase mediates ammonium homeostasis and is important for the polar growth of root hairs in Arabidopsis
The Arabidopsis receptor-like kinase CAP 1 promotes shoot growth under ammonium stress
ROP-GEF signal transduction is involved in AtCAP1-regulated root hair growth
Brassica juncea plant cadmium resistance 1 protein (BjPCR1) facilitates the radial transport of calcium in the root
Proceedings of the National Academy of Sciences of the United States of America,
Calcium/calmodulin- mediated microbial symbiotic interactions in plants
FERONIA as an upstream receptor kinase for polar cell growth in plants
Proceedings of the National Academy of Sciences of the United States of America,
A novel calmodulin-interacting Domain of Unknown Function 506 protein represses root hair elongation in Arabidopsis
MYB30 and ETHYLENE INSENEITIVE 3 antagonistically regulate root hair growth and phosphorus uptake under phosphate deficiency in Arabidopsis
Ethylene signalling is involved in regulation of phosphate starvation-induced gene expression and production of acid phosphatases and anthocyanin in Arabidopsis
Phosphate nutrition: improving low-phosphate tolerance in crops
The molecular mechanism of ethylene-mediated root hair development induced by phosphate starvation
Uncovering genes and ploidy involved in the high diversity in root hair density, length and response to local scarce phosphate in Arabidopsis thaliana
Root developmental adaptation to phosphate starvation:better safe than sorry
Nutrient-hormone relations: Driving root plasticity in plants
A mechanistic framework for auxin dependent Arabidopsis root hair elongation to low external phosphate
DOI:10.1038/s41467-018-03851-3
PMID:29651114
[本文引用: 1]

Phosphate (P) is an essential macronutrient for plant growth. Roots employ adaptive mechanisms to forage for P in soil. Root hair elongation is particularly important since P is immobile. Here we report that auxin plays a critical role promoting root hair growth in Arabidopsis in response to low external P. Mutants disrupting auxin synthesis (taa1) and transport (aux1) attenuate the low P root hair response. Conversely, targeting AUX1 expression in lateral root cap and epidermal cells rescues this low P response in aux1. Hence auxin transport from the root apex to differentiation zone promotes auxin-dependent hair response to low P. Low external P results in induction of root hair expressed auxin-inducible transcription factors ARF19, RSL2, and RSL4. Mutants lacking these genes disrupt the low P root hair response. We conclude auxin synthesis, transport and response pathway components play critical roles regulating this low P root adaptive response.
High auxin and high phosphate impact on RSL 2 expression and ROS- homeostasis linked to root hair growth in Arabidopsis thaliana
A phosphorus-limitation induced,functionally conserved DUF 506 protein is a repressor of root hair elongation in plants
The Ca2+-binding protein PCaP2 located on the plasma membrane is involved in root hair development as a possible signal transducer
Arabidopsis PCaP2 modulates the phosphatidylinositol 4,5-bisphosphate signal on the plasma membrane and attenuates root hair elongation
DOI:10.1111/tpj.14226
PMID:30604455
[本文引用: 1]

Phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P ] serves as a subcellular signal on the plasma membrane, mediating various cell-polarized phenomena including polar cell growth. Here, we investigated the involvement of Arabidopsis thaliana PCaP2, a plant-unique plasma membrane protein with phosphoinositide-binding activity, in PtdIns(4,5)P signaling for root hair tip growth. The long-root-hair phenotype of the pcap2 knockdown mutant was found to stem from its higher average root hair elongation rate compared with the wild type and to counteract the low average rate caused by a defect in the PtdIns(4,5)P -producing enzyme gene PIP5K3. On the plasma membrane of elongating root hairs, the PCaP2 promoter-driven PCaP2-green fluorescent protein (GFP), which complemented the pcap2 mutant phenotype, overlapped with the PtdIns(4,5)P marker 2xCHERRY-2xPH in the subapical region, but not at the apex, suggesting that PCaP2 attenuates root hair elongation via PtdIns(4,5)P signaling on the subapical plasma membrane. Consistent with this, a GFP fusion with the PCaP2 phosphoinositide-binding domain PCaP2, root hair-specific overexpression of which caused a low average root hair elongation rate, localized more intense to the subapical plasma membrane than to the apical plasma membrane similar to PCaP2-GFP. Inducibly overexpressed PCaP2-GFP, but not its derivative lacking the PCaP2 domain, replaced 2xCHERRY-2xPH on the plasma membrane in root meristematic epidermal cells, and suppressed FM4-64 internalization in elongating root hairs. Moreover, inducibly overexpressed PCaP2 arrested an endocytic process of PIN2-GFP recycling. Based on these results, we conclude that PCaP2 functions as a negative modulator of PtdIns(4,5)P signaling on the subapical plasma membrane probably through competitive binding to PtdIns(4,5)P and attenuates root hair elongation.© 2019 The Authors The Plant Journal © 2019 John Wiley & Sons Ltd.
Auxin, the organizer of the hormonal/ environmental signals for root hair growth
Molecular link between auxin and ROS-mediated polar growth
Proceedings of the National Academy of Sciences of the United States of America,
The rhd6 mutation of Arabidopsis thaliana alters root-hair initiation through an auxin- and ethylene- associated process
DOI:10.1104/pp.106.4.1335
PMID:12232412
[本文引用: 2]

Root-hair initiation in Arabidopsis thaliana provides a model for studying cell polarity and its role in plant morphogenesis. Root hairs normally emerge at the apical end of root epidermal cells, implying that these cells are polarized. We have identified a mutant, rhd6, that displays three defects: (a) a reduction in the number of root hairs, (b) an overall basal shift in the site of root-hair emergence, and (c) a relatively high frequency of epidermal cells with multiple root hairs. These defects implicate the RHD6 gene in root-hair initiation and indicate that RHD6 is normally associated with the establishment of, or response to, root epidermal cell polarity. Similar alterations in the site of root-hair emergence, although less extreme, were also discovered in roots of the auxin-, ethylene-, abscisic acid-resistant mutant axr2 and the ethylene-resistant mutant etr1. All three rhd6 mutant phenotypes were rescued when either auxin (indoleacetic acid) or an ethylene precursor (1-aminocyclopropane-1-carboxylic acid) was included in the growth medium. The rhd6 root phenotypes could be phenocopied by treating wild-type seedlings with an inhibitor of the ethylene pathway (aminoethoxyvinylglycine). These results indicate that RHD6 is normally involved in directing the selection or assembly of the root-hair initiation site through a process involving auxin and ethylene.
Hormones act downstream of TTG and GL2 to promote root hair outgrowth during epidermis development in the Arabidopsis root
DOI:10.1105/tpc.8.9.1505
PMID:8837505
[本文引用: 1]

The Arabidopsis root produces a position-dependent pattern of hair-bearing and hairless cell types during epidermis development. Five loci (TRANSPARENT TESTA GLABRA [TTG], GLABRA2 [GL2], ROOT HAIR DEFECTIVE6 [RHD6], CONSTITUTIVE TRIPLE RESPONSE1 [CTR1], and AUXIN RESISTANT2 [AXR2]) and the plant hormones ethylene and auxin have been reported to affect the production of root hair and hairless cells in the Arabidopsis root. In this study, genetic, molecular, and physiological tests were employed to define the roles of these loci and hormones. Epistasis tests and reporter gene studies indicated that the hairless cell-promoting genes TTG and GL2 are likely to act early to negatively regulate the ethylene and auxin pathways. Studies of the developmental timing of the hormone effects indicated that ethylene and auxin pathways promote root hair outgrowth after cell-type differentiation has been initiated. The genetic analysis of ethylene-and auxin-related mutations showed that root hair formation is influenced by a network of hormone pathways, including a partially redundant ethylene signaling pathway. A model is proposed in which the patterning of root epidermal cells in Arabidopsis is regulated by the cell position-dependent action of the TTG/GL2 pathway, and the ethylene and auxin hormone pathways act to promote root hair outgrowth at a relatively late stage of differentiation.
Developmental roles of AUX1/LAX auxin influx carriers in plants
DOI:10.3389/fpls.2019.01306
PMID:31719828
[本文引用: 1]

Plant hormone auxin regulates several aspects of plant growth and development. Auxin is predominantly synthesized in the shoot apex and developing leaf primordia and from there it is transported to the target tissues e.g. roots. Auxin transport is polar in nature and is carrier-mediated. AUXIN1/LIKE-AUX1 (AUX1/LAX) family members are the major auxin influx carriers whereas PIN-FORMED (PIN) family and some members of the P-GLYCOPROTEIN/ATP-BINDING CASSETTE B4 (PGP/ABCB) family are major auxin efflux carriers. AUX1/LAX auxin influx carriers are multi-membrane spanning transmembrane proteins sharing similarity to amino acid permeases. Mutations in genes result in auxin related developmental defects and have been implicated in regulating key plant processes including root and lateral root development, root gravitropism, root hair development, vascular patterning, seed germination, apical hook formation, leaf morphogenesis, phyllotactic patterning, female gametophyte development and embryo development. Recently AUX1 has also been implicated in regulating plant responses to abiotic stresses. This review summarizes our current understanding of the developmental roles of gene family and will also briefly discuss the modelling approaches that are providing new insight into the role of auxin transport in plant development.Copyright © 2019 Swarup and Bhosale.
AUX/LAX family of auxin influx carriers-an overview
DOI:10.3389/fpls.2012.00225
PMID:23087694
[本文引用: 1]

Auxin regulates several aspects of plant growth and development. Auxin is unique among plant hormones for exhibiting polar transport. Indole-3-acetic acid (IAA), the major form of auxin in higher plants, is a weak acid and its intercellular movement is facilitated by auxin influx and efflux carriers. Polarity of auxin movement is provided by asymmetric localization of auxin carriers (mainly PIN efflux carriers). PIN-FORMED (PIN) and P-GLYCOPROTEIN (PGP) family of proteins are major auxin efflux carriers whereas AUXIN1/LIKE-AUX1 (AUX/LAX) are major auxin influx carriers. Genetic and biochemical evidence show that each member of the AUX/LAX family is a functional auxin influx carrier and mediate auxin related developmental programmes in different organs and tissues. Of the four AUX/LAX genes, AUX1 regulates root gravitropism, root hair development and leaf phyllotaxy whereas LAX2 regulates vascular development in cotyledons. Both AUX1 and LAX3 have been implicated in lateral root (LR) development as well as apical hook formation whereas both AUX1 and LAX1 and possibly LAX2 are required for leaf phyllotactic patterning.
Transcriptional responses to the auxin hormone
DOI:10.1146/annurev-arplant-043015-112122
PMID:26905654
[本文引用: 1]

Auxin is arguably the most important signaling molecule in plants, and the last few decades have seen remarkable breakthroughs in understanding its production, transport, and perception. Recent investigations have focused on transcriptional responses to auxin, providing novel insight into the functions of the domains of key transcription regulators in responses to the hormonal cue and prominently implicating chromatin regulation in these responses. In addition, studies are beginning to identify direct targets of the auxin-responsive transcription factors that underlie auxin modulation of development. Mechanisms to tune the response to different auxin levels are emerging, as are first insights into how this single hormone can trigger diverse responses. Key unanswered questions center on the mechanism for auxin-directed transcriptional repression and the identity of additional determinants of auxin response specificity. Much of what has been learned in model plants holds true in other species, including the earliest land plants.
AUX1-mediated root hair auxin influx governs SCFTIR1/AFB-type Ca2+ signaling
AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues
High-affinity auxin transport by the AUX1 influx carrier protein
DOI:10.1016/j.cub.2006.04.029
PMID:16677815
[本文引用: 1]

In plants, auxin is a key regulator of development and is unique among plant hormones in that its function requires polarized transport between neighboring cells to form concentration gradients across various plant tissues. Although putative auxin-influx and -efflux transporters have been identified by using molecular genetic approaches, a detailed functional understanding for many of these transporters remains undetermined. Here we present the functional characterization of the auxin-influx carrier AUX1. Upon expression of AUX1 in Xenopus oocytes, saturable, pH-dependent uptake of 3H-IAA was measured. Mutations in AUX1 that abrogate physiological responses to IAA in planta resulted in loss or reduction of 3H-IAA uptake in AUX1-expressing oocytes. AUX1-mediated uptake of 3H-IAA was reduced by the IAA analogs 2,4-D and 1-NOA, but not by other auxin analogs. The measured Km for AUX1-mediated uptake of 3H-IAA was at concentrations at which physiological responses are observed for exogenously added IAA and 2,4-D. This is the first report demonstrating detailed functional characteristics of a plant auxin-influx transporter. This biochemical characterization provides new insights and a novel tool for studying auxin entry into cells and its pivotal roles in plant growth and development.
EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis
Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF
The plant hormone auxin is transported in a polar manner along the shoot-root axis, which requires efflux carriers such as PIN1. Asymmetric localization of PIN1 develops from a random distribution in Arabidopsis early embryogenesis. Coordinated polar localization of PIN1 is defective in gnom embryos. GNOM is a membrane-associated guanine-nucleotide exchange factor on ADP-ribosylation factor G protein (ARF GEF). Thus, GNOM-dependent vesicle trafficking may establish cell polarity, resulting in polar auxin transport.
Vectorial information for Arabidopsis planar polarity is mediated by combined AUX1, EIN2, and GNOM activity
Cell polarity is commonly coordinated within the plane of a single tissue layer (planar polarity), and hair positioning has been exploited as a simple marker for planar polarization of animal epithelia. The root epidermis of the plant Arabidopsis similarly reveals planar polarity of hair localization close to root tip-oriented (basal) ends of hair-forming cells. Hair position is directed toward a concentration maximum of the hormone auxin in the root tip, but mechanisms driving this plant-specific planar polarity remain elusive. Here, we report that combinatorial action of the auxin influx carrier AUX1, ETHYLENE-INSENSITIVE2 (EIN2), and GNOM genes mediates the vector for coordinate hair positioning. In aux1;ein2;gnom eb triple mutant roots, hairs display axial (apical or basal) instead of coordinate polar (basal) position, and recruitment of Rho-of-Plant (ROP) GTPases to the hair initiation site reveals the same polar-to-axial switch. The auxin concentration gradient is virtually abolished in aux1;ein2;gnom eb roots, where locally applied auxin can coordinate hair positioning. Moreover, auxin overproduction in sectors of wild-type roots enhances planar ROP and hair polarity over long and short distances. Hence, auxin may provide vectorial information for planar polarity that requires combinatorial AUX1, EIN2, and GNOM activity upstream of ROP positioning.
Rice auxin influx carrier OsAUX1 facilitates root hair elongation in response to low external phosphate
DOI:10.1038/s41467-018-03850-4
PMID:29650967
[本文引用: 2]

Root traits such as root angle and hair length influence resource acquisition particularly for immobile nutrients like phosphorus (P). Here, we attempted to modify root angle in rice by disrupting the OsAUX1 auxin influx transporter gene in an effort to improve rice P acquisition efficiency. We show by X-ray microCT imaging that root angle is altered in the osaux1 mutant, causing preferential foraging in the top soil where P normally accumulates, yet surprisingly, P acquisition efficiency does not improve. Through closer investigation, we reveal that OsAUX1 also promotes root hair elongation in response to P limitation. Reporter studies reveal that auxin response increases in the root hair zone in low P environments. We demonstrate that OsAUX1 functions to mobilize auxin from the root apex to the differentiation zone where this signal promotes hair elongation when roots encounter low external P. We conclude that auxin and OsAUX1 play key roles in promoting root foraging for P in rice.
The auxin influx carrier, OsAUX3, regulates rice root development and responses to aluminium stress
A transportome-scale amiRNA-based screen identifies redundant roles of Arabidopsis ABCB6 and ABCB 20 in auxin transport
P-glycoprotein4 displays auxin efflux transporter-like action in Arabidopsis root hair cells and tobacco cells
Cellular and molecular requirements for polar PIN targeting and transcytosis in plants
DOI:10.1093/mp/ssn062
PMID:19825603
[本文引用: 1]

The polar, sub-cellular localization of PIN auxin efflux carriers determines the direction of intercellular auxin flow, thus defining the spatial aspect of auxin signalling. Dynamic, transcytosis-like relocalizations of PIN proteins occur in response to external and internal signals, integrating these signals into changes in auxin distribution. Here, we examine the cellular and molecular mechanisms of polar PIN delivery and transcytosis. The mechanisms of the ARF-GEF-dependent polar targeting and transcytosis are well conserved and show little variations among diverse Arabidopsis ecotypes consistent with their fundamental importance in regulating plant development. At the cellular level, we refine previous findings on the role of the actin cytoskeleton in apical and basal PIN targeting, and identify a previously unknown role for microtubules, specifically in basal targeting. PIN protein delivery to different sides of the cell is mediated by ARF-dependent trafficking with a previously unknown complex level of distinct ARF-GEF vesicle trafficking regulators. Our data suggest that alternative recruitment of PIN proteins by these distinct pathways can account for cell type- and cargo-specific aspects of polar targeting, as well as for polarity changes in response to different signals. The resulting dynamic PIN positioning to different sides of cells defines a three-dimensional pattern of auxin fluxes within plant tissues.
SAV4 is required for ethylene-induced root hair growth through stabilizing PIN2 auxin transporter in Arabidopsis
Differential auxin-transporting activities of PIN-FORMED proteins in Arabidopsis root hair cells
DOI:10.1104/pp.110.156505
PMID:20439545
[本文引用: 2]

The Arabidopsis (Arabidopsis thaliana) genome includes eight PIN-FORMED (PIN) members that are molecularly diverged. To comparatively examine their differences in auxin-transporting activity and subcellular behaviors, we expressed seven PIN proteins specifically in Arabidopsis root hairs and analyzed their activities in terms of the degree of PIN-mediated root hair inhibition or enhancement and determined their subcellular localization. Expression of six PINs (PIN1-PIN4, PIN7, and PIN8) in root hair cells greatly inhibited root hair growth, most likely by lowering auxin levels in the root hair cell by their auxin efflux activities. The auxin efflux activity of PIN8, which had not been previously demonstrated, was further confirmed using a tobacco (Nicotiana tabacum) cell assay system. In accordance with these results, those PINs were localized in the plasma membrane, where they likely export auxin to the apoplast and formed internal compartments in response to brefeldin A. These six PINs conferred different degrees of root hair inhibition and sensitivities to auxin or auxin transport inhibitors. Conversely, PIN5 mostly localized to internal compartments, and its expression in root hair cells rather slightly stimulated hair growth, implying that PIN5 enhanced internal auxin availability. These results suggest that different PINs behave differentially in catalyzing auxin transport depending upon their molecular activity and subcellular localization in the root hair cell.
Intracellular auxin transport in pollen: PIN8, PIN5 and PILS5
Role of the Arabidopsis PIN6 auxin transporter in auxin homeostasis and auxin-mediated development
Ca2+ signals in plant immunity
NADase and now Ca2+ channel, what else to learn about plant NLRs?
DOI:10.1007/s44154-021-00007-0
PMID:37676511
[本文引用: 1]

Plant intracellular immune receptors known as NLR (Nucleotide-binding Leucine-rich repeat, NB-LRR) proteins confer resistance and cause cell death upon recognition of cognate effector proteins from pathogens. Plant NLRs contain a variable N-terminal domain: a Toll/interleukin-1 receptor (TIR) domain or a coiled-coil (CC) domain or an RPW8 (Resistance to Powdery Mildew 8)-like CC (CC) domain. TIR-NLR, CC-NLR and CC-NLR are known as TNL, CNL and RNL, respectively. TNLs and CNLs recognize pathogen effectors to activate cell death and defense responses, thus are regarded as sensor NLRs. RNLs are required downstream of TNLs to activate cell death and defense responses, thus are regarded as helper NLRs. Previous studies show that some TNLs form tetrameric resistosome as NAD cleaving enzymes to transduce signal, while some CNLs form pentameric resistosome with undefined biochemical function. Two recent breakthrough studies show that activated CNL and RNL function as Ca channel to cause cell death and defense responses and provide a completely new insight into the downstream signaling events of CNL and TNL pathways.© 2021. The Author(s).
Plasma membrane- associated calcium signaling modulates cadmium transport
Ethylene and plant responses to phosphate deficiency
DOI:10.3389/fpls.2015.00796
PMID:26483813
[本文引用: 1]

Phosphorus is an essential macronutrient for plant growth and development. Phosphate (Pi), the major form of phosphorus that plants take up through roots, however, is limited in most soils. To cope with Pi deficiency, plants activate an array of adaptive responses to reprioritize internal Pi use and enhance external Pi acquisition. These responses are modulated by sophisticated regulatory networks through both local and systemic signaling, but the signaling mechanisms are poorly understood. Early studies suggested that the phytohormone ethylene plays a key role in Pi deficiency-induced remodeling of root system architecture. Recently, ethylene was also shown to be involved in the regulation of other signature responses of plants to Pi deficiency. In this article, we review how researchers have used pharmacological and genetic approaches to dissect the roles of ethylene in regulating Pi deficiency-induced developmental and physiological changes. The interactions between ethylene and other signaling molecules, such as sucrose, auxin, and microRNA399, in the control of plant Pi responses are also examined. Finally, we provide a perspective for the future research in this field.
A model analysis of mechanisms for radial microtubular patterns at root hair initiation sites
Plant cells have two main modes of growth generating anisotropic structures. Diffuse growth where whole cell walls extend in specific directions, guided by anisotropically positioned cellulose fibers, and tip growth, with inhomogeneous addition of new cell wall material at the tip of the structure. Cells are known to regulate these processes via molecular signals and the cytoskeleton. Mechanical stress has been proposed to provide an input to the positioning of the cellulose fibers via cortical microtubules in diffuse growth. In particular, a stress feedback model predicts a circumferential pattern of fibers surrounding apical tissues and growing primordia, guided by the anisotropic curvature in such tissues. In contrast, during the initiation of tip growing root hairs, a star-like radial pattern has recently been observed. Here, we use detailed finite element models to analyze how a change in mechanical properties at the root hair initiation site can lead to star-like stress patterns in order to understand whether a stress-based feedback model can also explain the microtubule patterns seen during root hair initiation. We show that two independent mechanisms, individually or combined, can be sufficient to generate radial patterns. In the first, new material is added locally at the position of the root hair. In the second, increased tension in the initiation area provides a mechanism. Finally, we describe how a molecular model of Rho-of-plant (ROP) GTPases activation driven by auxin can position a patch of activated ROP protein basally along a 2D root epidermal cell plasma membrane, paving the way for models where mechanical and molecular mechanisms cooperate in the initial placement and outgrowth of root hairs.
Auxin and ROP GTPase signaling of polar nuclear migration in root epidermal hair cells
DOI:10.1104/pp.17.00713
PMID:29084900
[本文引用: 1]

Polar nuclear migration is crucial during the development of diverse eukaryotes. In plants, root hair growth requires polar nuclear migration into the outgrowing hair. However, knowledge about the dynamics and the regulatory mechanisms underlying nuclear movements in root epidermal cells remains limited. Here, we show that both auxin and Rho-of-Plant (ROP) signaling modulate polar nuclear position at the inner epidermal plasma membrane domain oriented to the cortical cells during cell elongation as well as subsequent polar nuclear movement to the outer domain into the emerging hair bulge in Arabidopsis (). Auxin signaling via the nuclear AUXIN RESPONSE FACTOR7 (ARF7)/ARF19 and INDOLE ACETIC ACID7 pathway ensures correct nuclear placement toward the inner membrane domain. Moreover, precise inner nuclear placement relies on SPIKE1 Rho-GEF, SUPERCENTIPEDE1 Rho-GDI, and ACTIN7 (ACT7) function and to a lesser extent on VTI11 vacuolar SNARE activity. Strikingly, the directionality and/or velocity of outer polar nuclear migration into the hair outgrowth along actin strands also are ACT7 dependent, auxin sensitive, and regulated by ROP signaling. Thus, our findings provide a founding framework revealing auxin and ROP signaling of inner polar nuclear position with some contribution by vacuolar morphology and of actin-dependent outer polar nuclear migration in root epidermal hair cells.© 2018 The author(s). All Rights Reserved.
Auxin controlled by ethylene steers root development