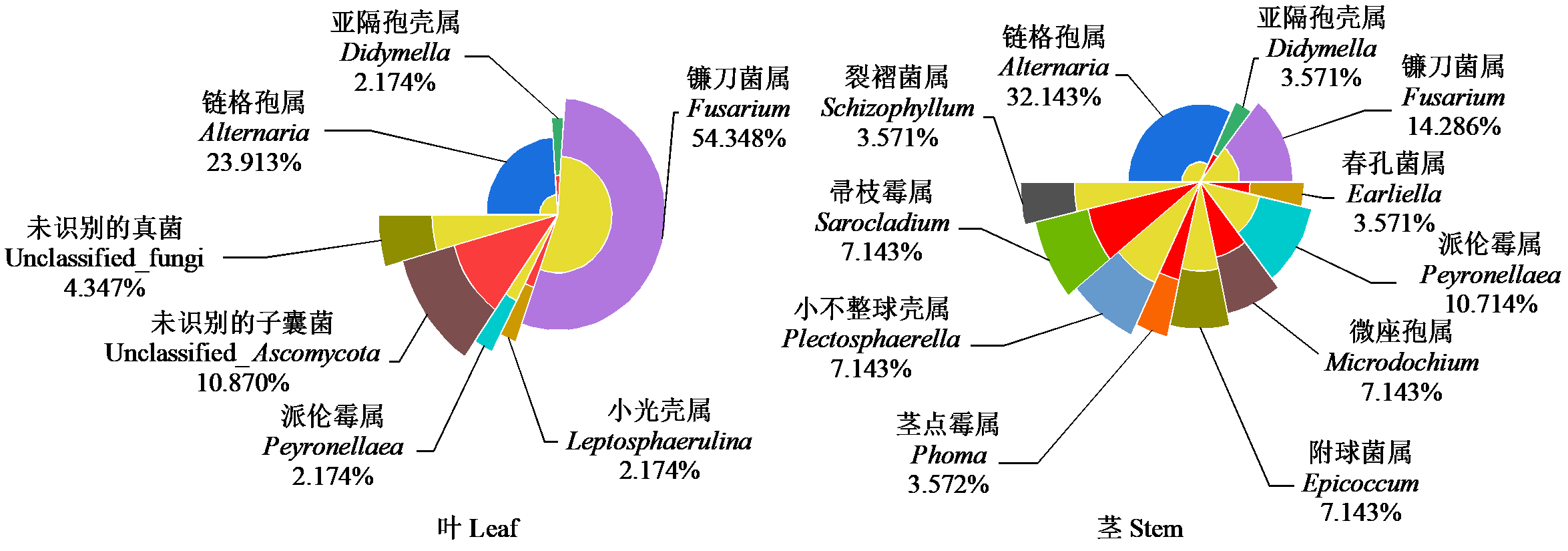作物杂志,2024, 第6期: 194–204 doi: 10.16035/j.issn.1001-7283.2024.06.026
燕麦地上部可培养内生真菌多样性分析及其功能研究
李晓婷1( ), 张婷婷2, 张艳丽1, 李志伟1, 韩丽1, 赵鑫瑶1, 张永平1, 李立军1(
), 张婷婷2, 张艳丽1, 李志伟1, 韩丽1, 赵鑫瑶1, 张永平1, 李立军1( )
)
- 1内蒙古农业大学农学院,010019,内蒙古呼和浩特
2乌兰察布市农林科学研究所,012000,内蒙古乌兰察布
Diversity Analysis and Function Study of Culturable Endophytic Fungi in Oat Shoot
Li Xiaoting1( ), Zhang Tingting2, Zhang Yanli1, Li Zhiwei1, Han Li1, Zhao Xinyao1, Zhang Yongping1, Li Lijun1(
), Zhang Tingting2, Zhang Yanli1, Li Zhiwei1, Han Li1, Zhao Xinyao1, Zhang Yongping1, Li Lijun1( )
)
- 1Agricultural College of Inner Mongolia Agricultural University, Hohhot 010019, Inner Mongolia, China
2Ulanqab Institute of Agriculture and Forestry Sciences, Ulanqab 012000, Inner Mongolia, China
摘要:
为探究燕麦叶和茎中可培养内生真菌分布情况、群落组成及其功能特性,通过组织培养分离法对阴山北麓区燕麦拔节期叶片和茎的内生真菌分离纯化,利用ITS鉴定并分析其多样性,同时测定其溶磷、解钾和分泌生长素(IAA)能力。结果发现,燕麦的叶和茎中分离得到74株内生真菌,总定殖率为61.94%,总分离率为20.56%,经过分子鉴定属于2门12属,其中优势菌门为子囊菌门(Ascomycota),优势菌属为链格孢菌属(Alternaria,分离率=5.56%,分离频率=27.03%)和镰刀菌属(Fusarium,分离率=8.06%,分离频率=39.19%)。多样性分析发现,茎中分离的内生真菌多样性高于叶,其多样性指数和辛普森指数分别为3.51和0.78。相似性分析发现,叶和茎中内生真菌的相似系数为0.15。对分离得到的内生真菌的功能分析发现,79.73%的菌株具有溶磷能力,72.97%的菌株具有解钾能力,40.54%的菌株能够分泌IAA。根据内生真菌的功能将74株内生真菌分为4类,分别有41、5、21和7株菌株,最优第4类菌株大部分均具有溶磷、解钾能力,且分泌IAA的能力最强。燕麦地上部组织中的内生真菌物种较为丰富,可为内生真菌的应用提供菌种资源,不同内生真菌的功能差异较大,功能性较强菌株在农业生产中可能会发挥其潜在促生长特性。
| [1] |
Hardoim P R, van Overbeek L S, Berg G, et al. The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiology and Molecular Biology Reviews, 2015, 79(3):293-320.
doi: 10.1128/MMBR.00050-14 pmid: 26136581 |
| [2] | 曹艳. 冬青卫矛内生菌的分离及其活性代谢物研究. 杨凌:西北农林科技大学, 2016. |
| [3] | 卢占慧. 人参内生菌群落多样性及拮抗菌株的抑菌作用研究. 沈阳:沈阳农业大学, 2016. |
| [4] | 王志伟, 纪燕玲, 陈永敢. 植物内生菌研究及其科学意义. 微生物学通报, 2015, 42(2):349-363. |
| [5] | Ofek-Lalzar M, Gur Y, Ben-Moshe S, et al. Diversity of fungal endophytes in recent and ancient wheat ancestors Triticum dicoccoides and Aegilops sharonensis. Fems Microbiology Ecology, 2016, 92(10):152. |
| [6] | Yuan Z L, Zhang C L, Lin F C, et al. Identity, diversity, and molecular phylogeny of the endophytic mycobiota in the roots of rare wild rice (Oryza granulate) from a nature reserve in Yunnan, China. Applied and Environmental Microbiology, 2010, 76(5):1642-1652. |
| [7] | 辛赫文, 徐建强, 杨岚, 等. 河南省小麦根、茎部内生真菌多样性及平板拮抗活性研究. 植物病理学报, 2022, 52(6):1-5. |
| [8] | 秦华伟, 门兴元, 卢增斌, 等. 山东省不同地区玉米内生真菌的群落组成和多样性分析. 植物保护学报, 2020, 47:35-42. |
| [9] | Schulz B J E, Boyle C J C, Sieber T N. Microbial root endophytes. Heidelberg:Springer, 2006. |
| [10] |
Young C A, Hume D E, Mcculley R L. Forages and pastures symposium: fungal endophytes of tall fescue and perennial ryegrass: pasture friend or foe?. Journal of Animal Science, 2013, 91(5):2379-2394.
doi: 10.2527/jas.2012-5951 pmid: 23307839 |
| [11] |
Waqas M, Khan A L, Kamran M, et al. Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules, 2012, 17(9):10754-10773.
doi: 10.3390/molecules170910754 pmid: 22960869 |
| [12] | Adhikari P, Pandey A. Phosphate solubilization potential of endophytic fungi isolated from Taxus wallichiana Zucc. roots. Rhizosphere, 2019, 9:2-9. |
| [13] | 詹寿发, 卢丹妮, 毛花英, 等. 2株溶磷、解钾与产IAA的内生真菌菌株的筛选、鉴定及促生作用研究. 中国土壤与肥料, 2017(3):142-151. |
| [14] | 徐萌, 王金缘, 胡金丽, 等. 植物内生菌对大豆促生长和抗胁迫作用的研究进展. 大豆科学, 2017, 36(6):965-969,977. |
| [15] | Puri A, Padda K P, Chanway C P. Seedling growth promotion and nitrogen fixation by a bacterial endophyte Paenibacillus polymyxa P2b-2R and its GFP derivative in corn in a long-term trial. Symbiosis, 2016, 69(2):123-129. |
| [16] | Nouh F A. Endophytic fungi for sustainable agriculture. Microbial Biosystems, 2019, 4(1):31-44. |
| [17] |
Baron N C, Rigobelo E C. Endophytic fungi: a tool for plant growth promotion and sustainable agriculture. Mycology, 2022, 13(1):39-55.
doi: 10.1080/21501203.2021.1945699 pmid: 35186412 |
| [18] | 刘军, 刘艳明, 徐在超, 等. 檀香内生真菌多样性及其抗菌与促生特性的研究. 中国中药杂志, 2018, 43(17):3477-3483. |
| [19] |
Edwards K, Johnstone C, Thompson C. A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Research, 1991, 19(6):1349.
doi: 10.1093/nar/19.6.1349 pmid: 2030957 |
| [20] | Schoch C L, Seifert K A, Huhndorf S, et al. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(16):6241-6246. |
| [21] | 鲁如坤. 土壤农业化学分析方法. 北京: 中国农业科学技术出版社, 2000. |
| [22] | 罗娜, 周德明, 徐睿, 等. 降香黄檀、檀香根际解钾菌的筛选与活性研究. 热带作物学报, 2016, 37(5):964-970. |
| [23] | 刘丽辉, 蒋慧敏, 区宇程, 等. 南方野生稻内生细菌的分离鉴定及促生作用. 应用与环境生物学报, 2020, 26(5):1051-1058. |
| [24] |
罗鑫, 于存. 贵州马尾松内生真菌多样性. 菌物学报, 2021, 40(3):531-546.
doi: 10.13346/j.mycosystema.200251 |
| [25] | 贾彤, 曹苗文, 周永娜, 等. 庞泉沟自然保护区常见禾本科植物内生真菌分布及其影响因素. 生态学报, 2017, 37(4):1103-1110. |
| [26] | 白浩楠, 牛香, 王兵, 等. 毛竹扩展对鹿角杜鹃叶内生真菌群落多样性的影响. 生态学杂志, 2021, 40(12):3849-3859. |
| [27] | 刘永兰, 张丽娜, 梁路, 等. 贵州赤水桫椤自然保护区桫椤内生真菌多样性研究. 菌物学报, 2021, 40(12):1-12. |
| [28] | Vieira M L, Hughes A F, Gil V B, et al. Diversity and antimicrobial activities of the fungal endophyte community associated with the traditional Brazilian medicinal plant Solanum cernuum Vell. (Solanaceae). Canadian Journal of Microbiology, 2012, 58(1):54-66. |
| [29] |
Arnold A E, Miadlikowska J, Higgins K L, et al. A phylogenetic estimation of trophic transition networks for ascomycetous fungi: are lichens cradles of symbiotrophic fungal diversification?. Systematic Biology, 2009, 58(3):283-297.
doi: 10.1093/sysbio/syp001 pmid: 20525584 |
| [30] | 苗文莉. 小麦内生真菌多样性及其与宿主关系研究. 郑州:郑州大学, 2011. |
| [31] | 禹乐乐. 玉米内生真菌多样性及其与宿主关系研究. 郑州:郑州大学, 2012. |
| [32] | 潘文文. 卷柏内生真菌多样性及其对小麦抗旱性的影响. 郑州:郑州大学, 2016. |
| [33] |
Lofgren L A, Leblanc N R, Certano A K, et al. Fusarium graminearum: pathogen or endophyte of North American grasses?. New Phytologist, 2018, 217(3):1203-1212.
doi: 10.1111/nph.14894 pmid: 29160900 |
| [34] | Dai C C, Yu B Y, Li X. Screening of endophytic fungi that promote the growth of Euphorbia pekinensis. African Journal of Biotechnology, 2008, 7(19):3505-3510. |
| [35] | 代梦雪, 张光群, 范旭杪, 等. 胁迫生境深色有隔内生真菌生态分布与功能研究进展. 应用与环境生物学报, 2020, 26(3):722-729. |
| [36] |
Massimo N C, Nandi Devan M M, Arendt K R, et al. Fungal endophytes in aboveground tissues of desert plants: infrequent in culture, but highly diverse and distinctive symbionts. Microbial Ecology, 2015, 70(1):61-76.
doi: 10.1007/s00248-014-0563-6 pmid: 25645243 |
| [37] | Sadeghi F, Samsampour D, Seyahooei M A, et al. Diversity and spatiotemporal distribution of fungal endophytes associated with Citrus reticulata cv. Siyahoo. Current Microbiology, 2019, 76(3):279-289. |
| [38] | Sun X, Ding Q, Hyde K D, et al. Community structure and preference of endophytic fungi of three woody plants in a mixed forest. Fungal Ecology, 2012, 5(5):624-632. |
| [39] | Siddique A B, Biella P, Unterseher M, et al. Mycobiomes of young beech trees are distinguished by organ rather than by habitat, and community analyses suggest competitive interactions among twig fungi. Frontiers in Microbiology, 2021, 12:646302. |
| [40] |
Harrison J G, Griffin E A. The diversity and distribution of endophytes across biomes, plant phylogeny and host tissues: how far have we come and where do we go from here?. Environmental Microbiology, 2020, 22(6):2107-2123.
doi: 10.1111/1462-2920.14968 pmid: 32115818 |
| [41] | Yuan Z S, Liu F, Zhang G F. Characteristics and biodiversity of endophytic phosphorus- and potassium-solubilizing bacteria in Moso Bamboo (Phyllostachys edulis). Acta Biologica Hungarica, 2015, 66(4):449-459. |
| [42] | Singh B P. Advances in endophytic fungal research: present status and future challenges. Berlin,Germany:Springer, 2019. |
| [43] | 唐嘉城, 梁毅珉, 马葭思, 等. 百香果内生细菌多样性及促生长特性. 生物科技通报, 2022, 38(1):86-97. |
| [44] | Nath R, Sharma G D, Barooah M. Plant growth promoting endophytic fungi isolated from tea (Camellia Sinensis) shrubs of Assam, India. Applied Ecology and Environmental Research, 2015, 13(3):877-891. |
| [45] | Hassan E D. Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L.. Journal of Advanced Research, 2017, 8(6):687-695. |
| [46] | Liao X G, Lovett B, Fang W G, et al. Metarhizium robertsii produces indole-3-acetic acid, which promotes root growth in Arabidopsis and enhances virulence to insects. Microbiology, 2017, 163(7):980-991. |
| [1] | 李峰, 高宏云, 张翀, 张宝英, 马建富, 郭娜, 白苇, 方爱国, 杨志敏, 李源. 盐胁迫对燕麦生长及生理指标的影响[J]. 作物杂志, 2024, (6): 140–146 |
| [2] | 蒋凤林, 雷雄彪, 赵嘉暄, 黄敏, 李曼菲, 杜何为. 植物根毛生长发育过程及分子调控机制[J]. 作物杂志, 2024, (6): 9–17 |
| [3] | 毛向红, 范向斌, 白小东, 卢瑶, 杜培兵. 基于SSR分子标记的晋北地区引进马铃薯种质资源遗传多样性分析[J]. 作物杂志, 2024, (5): 54–59 |
| [4] | 袁迪, 智慧, 王海岗, 张慧, 姚琦, 梁红凯, 王君杰, 陈凌, 刁现民, 贾冠清. 我国谷子登记品种遗传多样性分析及综合评价[J]. 作物杂志, 2024, (4): 14–23 |
| [5] | 胡连清, 陈露, 刘雯雯, 周万海, 冯瑞章, 魏琴, 赵鑫, 舒豪, 陈玲妹, 陈雨薇. 酿酒专用糯红高粱内生细菌多样性分析及功能菌株筛选[J]. 作物杂志, 2024, (4): 194–202 |
| [6] | 李清超, 张登峰, 李春辉, 杨珊, 刘建新, 吴迅. 西南地区玉米地方种质资源遗传多样性分析及综合评价[J]. 作物杂志, 2024, (4): 24–32 |
| [7] | 范昱, 冯亮, 王俊珍, 杨乔惠, 任远航, 张凯旋, 邹亮, 周美亮, 向达兵. 不同品种燕麦的营养成分分析[J]. 作物杂志, 2024, (4): 71–81 |
| [8] | 代涵, 申铁, 石桃雄, 黎瑞源. 油茶基因组SSR位点挖掘及遗传多样性分析[J]. 作物杂志, 2024, (3): 23–31 |
| [9] | 马红珍, 许海涛, 王月, 冯晓曦, 许波, 张军刚, 郭海斌, 王友华. 基于苞叶表型性状的玉米自交系遗传多样性及遗传距离分析[J]. 作物杂志, 2024, (3): 54–63 |
| [10] | 全成哲, 李淑芳, 李鹤南, 于维, 金京花. 吉林省73份审定水稻品种的表型性状遗传多样性研究[J]. 作物杂志, 2024, (3): 64–75 |
| [11] | 孙悦颖, 刘景辉, 米俊珍, 赵宝平, 李英浩, 朱珊珊. 乳酸菌复合制剂对燕麦的促生作用研究[J]. 作物杂志, 2024, (2): 122–128 |
| [12] | 杨恩泽, 谢锐, 韩平安, 张永虎, 刘锦川, 牛素清, 温蕊, 王春勇, 金晓蕾. 内蒙古162份苦荞资源表型性状的遗传多样性及综合评价[J]. 作物杂志, 2024, (2): 15–22 |
| [13] | 陈林, 姚晓华, 姚有华, 白羿雄, 吴昆仑. 青藏高原青稞品种籽粒外观和品质性状的多样性分析[J]. 作物杂志, 2024, (2): 213–220 |
| [14] | 周镇磊, 刘建明, 曹东, 刘宝龙, 王东霞, 张怀刚. 不同燕麦品种草产量、农艺性状和饲草品质的比较[J]. 作物杂志, 2024, (1): 132–140 |
| [15] | 张璐, 李登明, 翟晓宇, 武俊英, 高世华, 赵宇飞. 燕麦刈割期农艺与品质性状差异及其与再生性能的关系[J]. 作物杂志, 2024, (1): 220–228 |
|
||